 Found 107 hits from Universitat Jena
Found 107 hits from Universitat Jena Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine protease 1
(Homo sapiens (Human)) | BDBM50110023
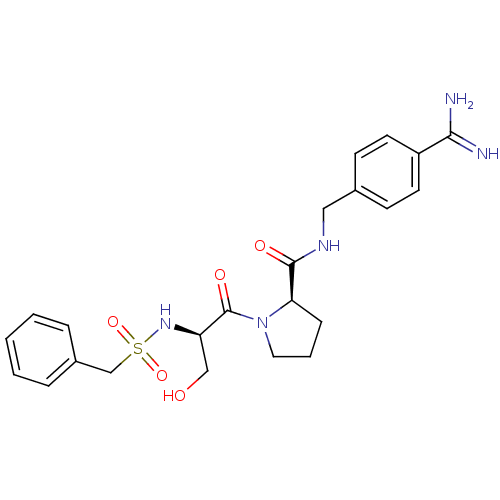
(2-(3-Hydroxy-2-phenylmethanesulfonylamino-propiony...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29N5O5S/c24-21(25)18-10-8-16(9-11-18)13-26-22(30)20-7-4-12-28(20)23(31)19(14-29)27-34(32,33)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,27,29H,4,7,12-15H2,(H3,24,25)(H,26,30)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110025
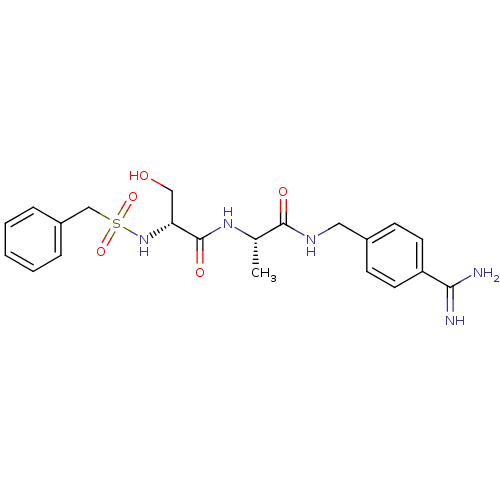
(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110016
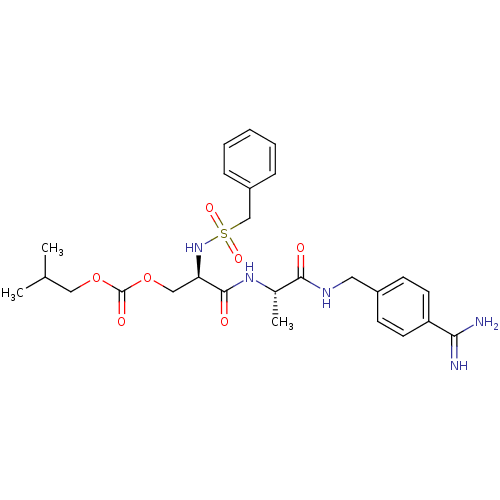
(CHEMBL433510 | Carbonic acid 2-[1-(4-carbamimidoyl...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H35N5O7S/c1-17(2)14-37-26(34)38-15-22(31-39(35,36)16-20-7-5-4-6-8-20)25(33)30-18(3)24(32)29-13-19-9-11-21(12-10-19)23(27)28/h4-12,17-18,22,31H,13-16H2,1-3H3,(H3,27,28)(H,29,32)(H,30,33)/t18-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50110023

(2-(3-Hydroxy-2-phenylmethanesulfonylamino-propiony...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29N5O5S/c24-21(25)18-10-8-16(9-11-18)13-26-22(30)20-7-4-12-28(20)23(31)19(14-29)27-34(32,33)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,27,29H,4,7,12-15H2,(H3,24,25)(H,26,30)/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of thrombin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110023

(2-(3-Hydroxy-2-phenylmethanesulfonylamino-propiony...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29N5O5S/c24-21(25)18-10-8-16(9-11-18)13-26-22(30)20-7-4-12-28(20)23(31)19(14-29)27-34(32,33)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,27,29H,4,7,12-15H2,(H3,24,25)(H,26,30)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110015
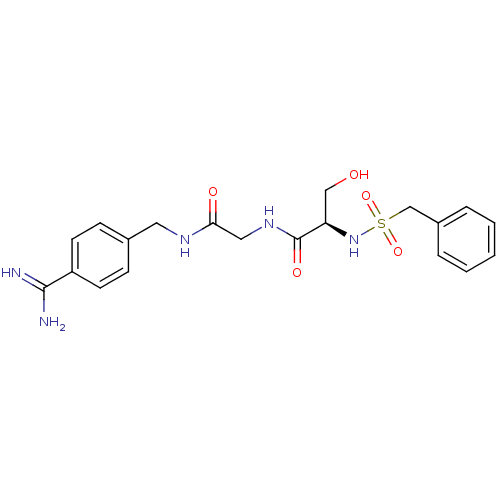
(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110016

(CHEMBL433510 | Carbonic acid 2-[1-(4-carbamimidoyl...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H35N5O7S/c1-17(2)14-37-26(34)38-15-22(31-39(35,36)16-20-7-5-4-6-8-20)25(33)30-18(3)24(32)29-13-19-9-11-21(12-10-19)23(27)28/h4-12,17-18,22,31H,13-16H2,1-3H3,(H3,27,28)(H,29,32)(H,30,33)/t18-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110014
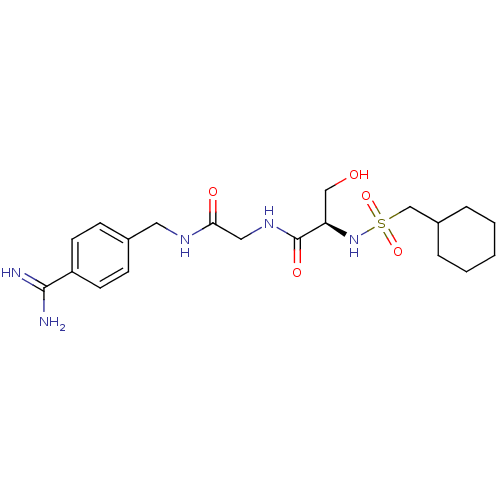
(CHEMBL158814 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)CC2CCCCC2)cc1 Show InChI InChI=1S/C20H31N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h6-9,15,17,25-26H,1-5,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50110016

(CHEMBL433510 | Carbonic acid 2-[1-(4-carbamimidoyl...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H35N5O7S/c1-17(2)14-37-26(34)38-15-22(31-39(35,36)16-20-7-5-4-6-8-20)25(33)30-18(3)24(32)29-13-19-9-11-21(12-10-19)23(27)28/h4-12,17-18,22,31H,13-16H2,1-3H3,(H3,27,28)(H,29,32)(H,30,33)/t18-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of thrombin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110019
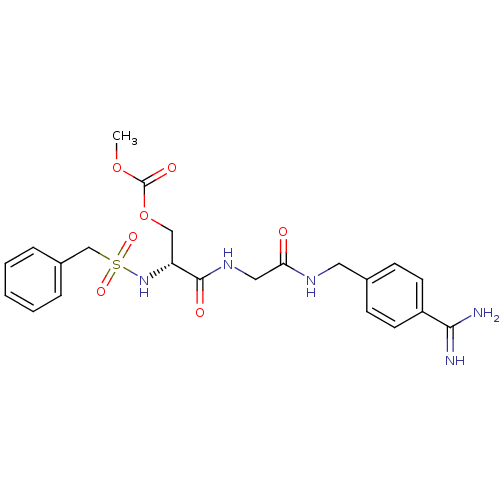
(CHEMBL159162 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-33-22(30)34-13-18(27-35(31,32)14-16-5-3-2-4-6-16)21(29)26-12-19(28)25-11-15-7-9-17(10-8-15)20(23)24/h2-10,18,27H,11-14H2,1H3,(H3,23,24)(H,25,28)(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of thrombin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110014

(CHEMBL158814 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)CC2CCCCC2)cc1 Show InChI InChI=1S/C20H31N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h6-9,15,17,25-26H,1-5,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110024
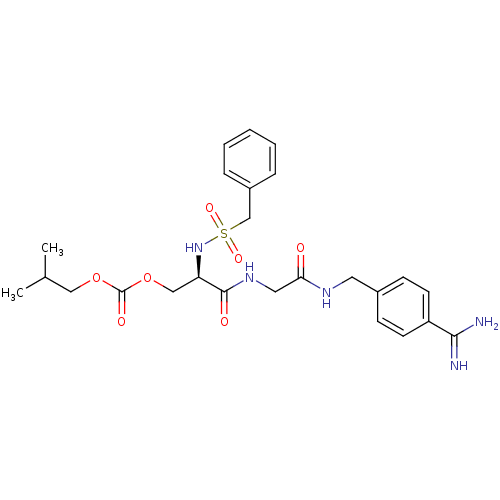
(CHEMBL345435 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C25H33N5O7S/c1-17(2)14-36-25(33)37-15-21(30-38(34,35)16-19-6-4-3-5-7-19)24(32)29-13-22(31)28-12-18-8-10-20(11-9-18)23(26)27/h3-11,17,21,30H,12-16H2,1-2H3,(H3,26,27)(H,28,31)(H,29,32)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50110023

(2-(3-Hydroxy-2-phenylmethanesulfonylamino-propiony...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29N5O5S/c24-21(25)18-10-8-16(9-11-18)13-26-22(30)20-7-4-12-28(20)23(31)19(14-29)27-34(32,33)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,27,29H,4,7,12-15H2,(H3,24,25)(H,26,30)/t19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Plasmin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110015

(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50110016

(CHEMBL433510 | Carbonic acid 2-[1-(4-carbamimidoyl...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H35N5O7S/c1-17(2)14-37-26(34)38-15-22(31-39(35,36)16-20-7-5-4-6-8-20)25(33)30-18(3)24(32)29-13-19-9-11-21(12-10-19)23(27)28/h4-12,17-18,22,31H,13-16H2,1-3H3,(H3,27,28)(H,29,32)(H,30,33)/t18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Plasmin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110019

(CHEMBL159162 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-33-22(30)34-13-18(27-35(31,32)14-16-5-3-2-4-6-16)21(29)26-12-19(28)25-11-15-7-9-17(10-8-15)20(23)24/h2-10,18,27H,11-14H2,1H3,(H3,23,24)(H,25,28)(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110019

(CHEMBL159162 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-33-22(30)34-13-18(27-35(31,32)14-16-5-3-2-4-6-16)21(29)26-12-19(28)25-11-15-7-9-17(10-8-15)20(23)24/h2-10,18,27H,11-14H2,1H3,(H3,23,24)(H,25,28)(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110016

(CHEMBL433510 | Carbonic acid 2-[1-(4-carbamimidoyl...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H35N5O7S/c1-17(2)14-37-26(34)38-15-22(31-39(35,36)16-20-7-5-4-6-8-20)25(33)30-18(3)24(32)29-13-19-9-11-21(12-10-19)23(27)28/h4-12,17-18,22,31H,13-16H2,1-3H3,(H3,27,28)(H,29,32)(H,30,33)/t18-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110010

(CHEMBL415741 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)CCc2ccccc2)cc1 Show InChI InChI=1S/C21H27N5O5S/c22-20(23)17-8-6-16(7-9-17)12-24-19(28)13-25-21(29)18(14-27)26-32(30,31)11-10-15-4-2-1-3-5-15/h1-9,18,26-27H,10-14H2,(H3,22,23)(H,24,28)(H,25,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110024

(CHEMBL345435 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C25H33N5O7S/c1-17(2)14-36-25(33)37-15-21(30-38(34,35)16-19-6-4-3-5-7-19)24(32)29-13-22(31)28-12-18-8-10-20(11-9-18)23(26)27/h3-11,17,21,30H,12-16H2,1-2H3,(H3,26,27)(H,28,31)(H,29,32)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110024

(CHEMBL345435 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C25H33N5O7S/c1-17(2)14-36-25(33)37-15-21(30-38(34,35)16-19-6-4-3-5-7-19)24(32)29-13-22(31)28-12-18-8-10-20(11-9-18)23(26)27/h3-11,17,21,30H,12-16H2,1-2H3,(H3,26,27)(H,28,31)(H,29,32)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Plasmin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110012
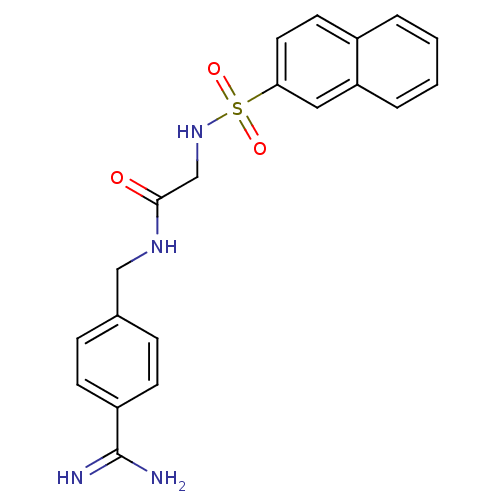
(CHEMBL156858 | N-(4-Carbamimidoyl-benzyl)-2-(napht...)Show SMILES NC(=N)c1ccc(CNC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C20H20N4O3S/c21-20(22)16-7-5-14(6-8-16)12-23-19(25)13-24-28(26,27)18-10-9-15-3-1-2-4-17(15)11-18/h1-11,24H,12-13H2,(H3,21,22)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110010

(CHEMBL415741 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)CCc2ccccc2)cc1 Show InChI InChI=1S/C21H27N5O5S/c22-20(23)17-8-6-16(7-9-17)12-24-19(28)13-25-21(29)18(14-27)26-32(30,31)11-10-15-4-2-1-3-5-15/h1-9,18,26-27H,10-14H2,(H3,22,23)(H,24,28)(H,25,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110008
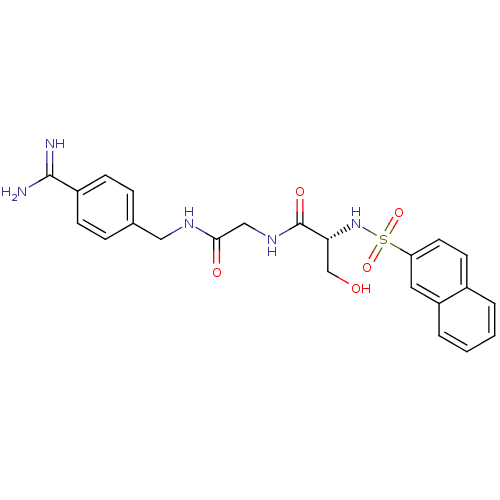
(CHEMBL346117 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C23H25N5O5S/c24-22(25)17-7-5-15(6-8-17)12-26-21(30)13-27-23(31)20(14-29)28-34(32,33)19-10-9-16-3-1-2-4-18(16)11-19/h1-11,20,28-29H,12-14H2,(H3,24,25)(H,26,30)(H,27,31)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110026
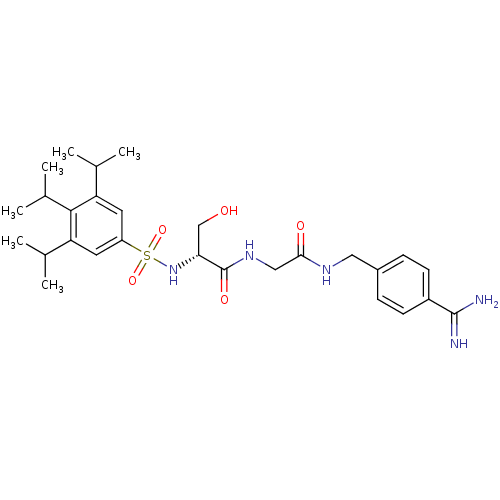
(CHEMBL157131 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES CC(C)c1cc(cc(C(C)C)c1C(C)C)S(=O)(=O)N[C@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H41N5O5S/c1-16(2)22-11-21(12-23(17(3)4)26(22)18(5)6)39(37,38)33-24(15-34)28(36)32-14-25(35)31-13-19-7-9-20(10-8-19)27(29)30/h7-12,16-18,24,33-34H,13-15H2,1-6H3,(H3,29,30)(H,31,35)(H,32,36)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110006
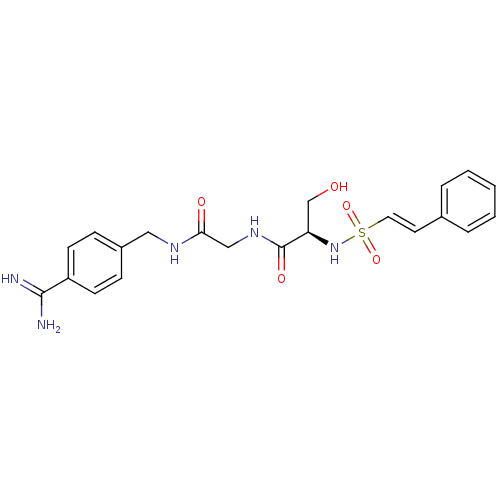
(CHEMBL352009 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C21H25N5O5S/c22-20(23)17-8-6-16(7-9-17)12-24-19(28)13-25-21(29)18(14-27)26-32(30,31)11-10-15-4-2-1-3-5-15/h1-11,18,26-27H,12-14H2,(H3,22,23)(H,24,28)(H,25,29)/b11-10+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110008

(CHEMBL346117 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C23H25N5O5S/c24-22(25)17-7-5-15(6-8-17)12-26-21(30)13-27-23(31)20(14-29)28-34(32,33)19-10-9-16-3-1-2-4-18(16)11-19/h1-11,20,28-29H,12-14H2,(H3,24,25)(H,26,30)(H,27,31)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110023

(2-(3-Hydroxy-2-phenylmethanesulfonylamino-propiony...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29N5O5S/c24-21(25)18-10-8-16(9-11-18)13-26-22(30)20-7-4-12-28(20)23(31)19(14-29)27-34(32,33)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,27,29H,4,7,12-15H2,(H3,24,25)(H,26,30)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110014

(CHEMBL158814 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)CC2CCCCC2)cc1 Show InChI InChI=1S/C20H31N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h6-9,15,17,25-26H,1-5,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110006

(CHEMBL352009 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C21H25N5O5S/c22-20(23)17-8-6-16(7-9-17)12-24-19(28)13-25-21(29)18(14-27)26-32(30,31)11-10-15-4-2-1-3-5-15/h1-11,18,26-27H,12-14H2,(H3,22,23)(H,24,28)(H,25,29)/b11-10+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50110024

(CHEMBL345435 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C25H33N5O7S/c1-17(2)14-36-25(33)37-15-21(30-38(34,35)16-19-6-4-3-5-7-19)24(32)29-13-22(31)28-12-18-8-10-20(11-9-18)23(26)27/h3-11,17,21,30H,12-16H2,1-2H3,(H3,26,27)(H,28,31)(H,29,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Plasmin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110007
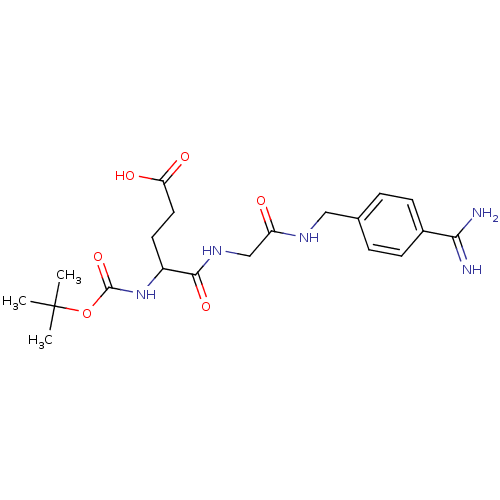
(4-tert-Butoxycarbonylamino-4-{[(4-carbamimidoyl-be...)Show SMILES CC(C)(C)OC(=O)NC(CCC(O)=O)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C20H29N5O6/c1-20(2,3)31-19(30)25-14(8-9-16(27)28)18(29)24-11-15(26)23-10-12-4-6-13(7-5-12)17(21)22/h4-7,14H,8-11H2,1-3H3,(H3,21,22)(H,23,26)(H,24,29)(H,25,30)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50110015

(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor Xa. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50110019

(CHEMBL159162 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-33-22(30)34-13-18(27-35(31,32)14-16-5-3-2-4-6-16)21(29)26-12-19(28)25-11-15-7-9-17(10-8-15)20(23)24/h2-10,18,27H,11-14H2,1H3,(H3,23,24)(H,25,28)(H,26,29)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of thrombin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50088982
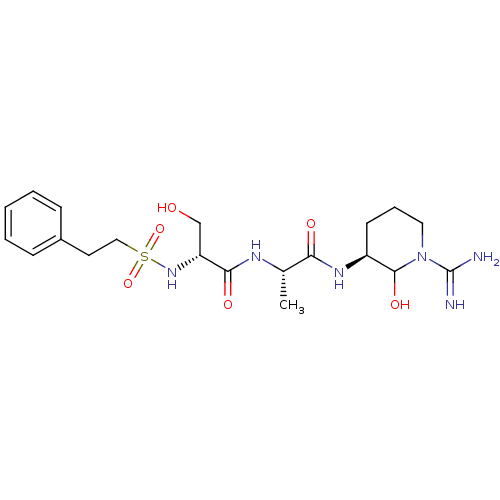
(CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoy...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)CCc1ccccc1)C(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C20H32N6O6S/c1-13(17(28)24-15-8-5-10-26(19(15)30)20(21)22)23-18(29)16(12-27)25-33(31,32)11-9-14-6-3-2-4-7-14/h2-4,6-7,13,15-16,19,25,27,30H,5,8-12H2,1H3,(H3,21,22)(H,23,29)(H,24,28)/t13-,15-,16+,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase after a 30 min incubation period. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110026

(CHEMBL157131 | N-[(4-Carbamimidoyl-benzylcarbamoyl...)Show SMILES CC(C)c1cc(cc(C(C)C)c1C(C)C)S(=O)(=O)N[C@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H41N5O5S/c1-16(2)22-11-21(12-23(17(3)4)26(22)18(5)6)39(37,38)33-24(15-34)28(36)32-14-25(35)31-13-19-7-9-20(10-8-19)27(29)30/h7-12,16-18,24,33-34H,13-15H2,1-6H3,(H3,29,30)(H,31,35)(H,32,36)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110013
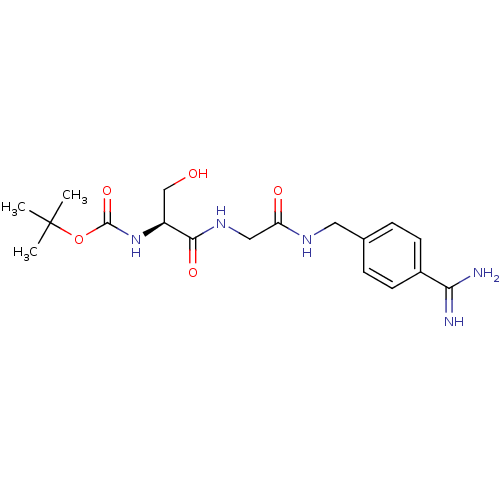
((1-{[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-car...)Show SMILES CC(C)(C)OC(=O)N[C@@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C18H27N5O5/c1-18(2,3)28-17(27)23-13(10-24)16(26)22-9-14(25)21-8-11-4-6-12(7-5-11)15(19)20/h4-7,13,24H,8-10H2,1-3H3,(H3,19,20)(H,21,25)(H,22,26)(H,23,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110005
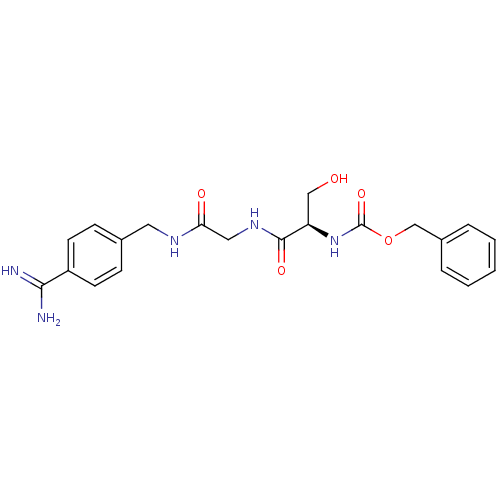
((1-{[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-car...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N5O5/c22-19(23)16-8-6-14(7-9-16)10-24-18(28)11-25-20(29)17(12-27)26-21(30)31-13-15-4-2-1-3-5-15/h1-9,17,27H,10-13H2,(H3,22,23)(H,24,28)(H,25,29)(H,26,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50110024

(CHEMBL345435 | Carbonic acid 2-{[(4-carbamimidoyl-...)Show SMILES CC(C)COC(=O)OC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C25H33N5O7S/c1-17(2)14-36-25(33)37-15-21(30-38(34,35)16-19-6-4-3-5-7-19)24(32)29-13-22(31)28-12-18-8-10-20(11-9-18)23(26)27/h3-11,17,21,30H,12-16H2,1-2H3,(H3,26,27)(H,28,31)(H,29,32)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of thrombin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110005

((1-{[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-car...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N5O5/c22-19(23)16-8-6-14(7-9-16)10-24-18(28)11-25-20(29)17(12-27)26-21(30)31-13-15-4-2-1-3-5-15/h1-9,17,27H,10-13H2,(H3,22,23)(H,24,28)(H,25,29)(H,26,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110004

((1-{[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-car...)Show SMILES CC(C)(C)OC(=O)N[C@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C18H27N5O5/c1-18(2,3)28-17(27)23-13(10-24)16(26)22-9-14(25)21-8-11-4-6-12(7-5-11)15(19)20/h4-7,13,24H,8-10H2,1-3H3,(H3,19,20)(H,21,25)(H,22,26)(H,23,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110011
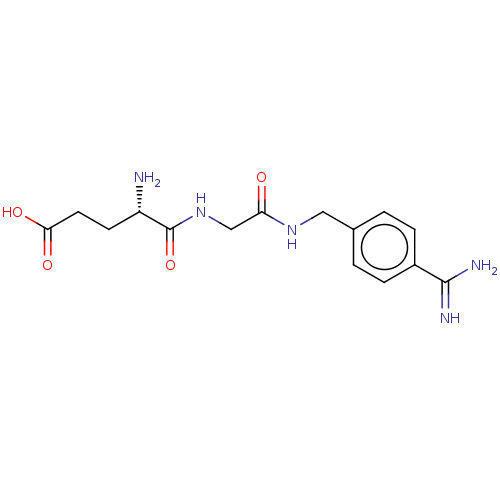
(4-Amino-4-{[(4-carbamimidoyl-benzylcarbamoyl)-meth...)Show SMILES N[C@@H](CCC(O)=O)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C15H21N5O4/c16-11(5-6-13(22)23)15(24)20-8-12(21)19-7-9-1-3-10(4-2-9)14(17)18/h1-4,11H,5-8,16H2,(H3,17,18)(H,19,21)(H,20,24)(H,22,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110018
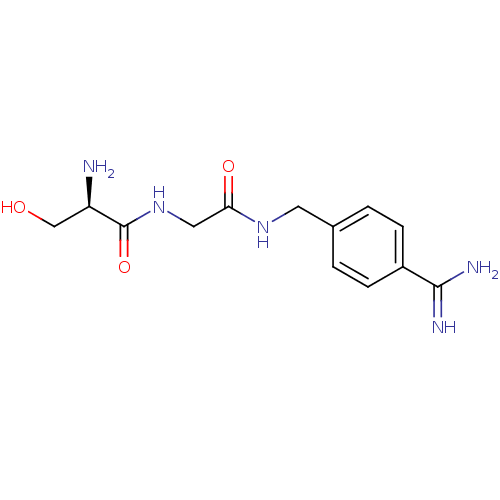
(2-Amino-N-[(4-carbamimidoyl-benzylcarbamoyl)-methy...)Show InChI InChI=1S/C13H19N5O3/c14-10(7-19)13(21)18-6-11(20)17-5-8-1-3-9(4-2-8)12(15)16/h1-4,10,19H,5-7,14H2,(H3,15,16)(H,17,20)(H,18,21)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50110017
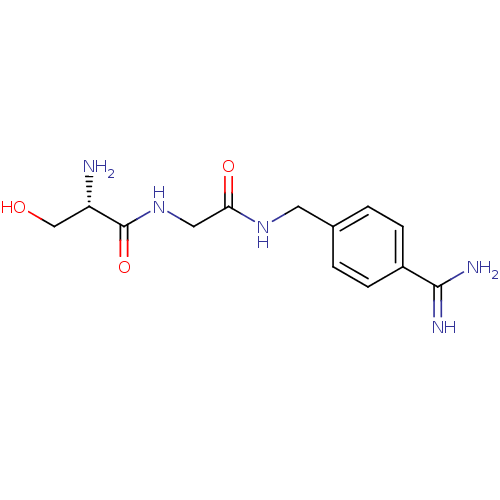
(2-Amino-N-[(4-carbamimidoyl-benzylcarbamoyl)-methy...)Show InChI InChI=1S/C13H19N5O3/c14-10(7-19)13(21)18-6-11(20)17-5-8-1-3-9(4-2-8)12(15)16/h1-4,10,19H,5-7,14H2,(H3,15,16)(H,17,20)(H,18,21)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of trypsin. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110004

((1-{[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-car...)Show SMILES CC(C)(C)OC(=O)N[C@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C18H27N5O5/c1-18(2,3)28-17(27)23-13(10-24)16(26)22-9-14(25)21-8-11-4-6-12(7-5-11)15(19)20/h4-7,13,24H,8-10H2,1-3H3,(H3,19,20)(H,21,25)(H,22,26)(H,23,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110012

(CHEMBL156858 | N-(4-Carbamimidoyl-benzyl)-2-(napht...)Show SMILES NC(=N)c1ccc(CNC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C20H20N4O3S/c21-20(22)16-7-5-14(6-8-16)12-23-19(25)13-24-28(26,27)18-10-9-15-3-1-2-4-17(15)11-18/h1-11,24H,12-13H2,(H3,21,22)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data